Navigate to ▼
- Clinical Trials
- Drug Safety and Pharmacovigilance
- Good Manufacturing Practice
- Good Practices for Regulated Laboratories
- Medical Devices
- Information & Communication Technology
- Pharmacokinetics and Pharmacodynamics
- Regulatory Affairs & Compliance
- Sales and Marketing
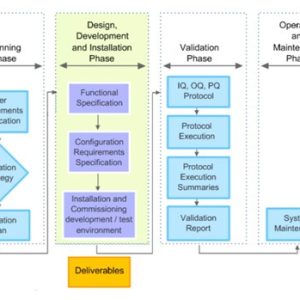
- Validation
- Micro Courses (CT & GMP)
PRICING AND DISCOUNTS
| Purchase | Discount |
|---|---|
| 1 course | 0% |
| 2 courses | 5% |
| 3 courses | 10% |
| 4 courses | 15% |
| 5 courses | 20% |
| more(each) | 25% |
Featured Products
-
ICH E6(R3) Good Clinical Practice
£159.00Original price was: £159.00.£99.00Current price is: £99.00. exc. VAT -
Good Clinical Laboratory Practice
£69.00Original price was: £69.00.£49.00Current price is: £49.00. exc. VAT -
An Introduction to Drug Safety and Pharmacovigilance
£149.00Original price was: £149.00.£139.00Current price is: £139.00. exc. VAT
Clinical Trials
-
How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
ICH E6(R3) Good Clinical Practice
£159.00Original price was: £159.00.£99.00Current price is: £99.00. exc. VAT -
An Introduction to Clinical Trial Preparation and Design
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
Clinical Trial Monitoring: Site Evaluation and Set-up
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
£98.00Original price was: £98.00.£75.00Current price is: £75.00. exc. VAT -
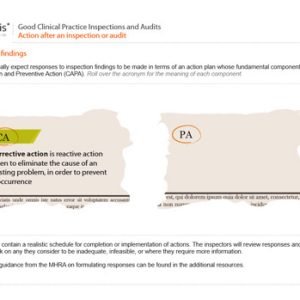
Good Clinical Practice Inspections and Audits
£126.00Original price was: £126.00.£89.00Current price is: £89.00. exc. VAT -
The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
How to Conduct Clinical Research Under the EU Clinical Trials Regulation
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Safety Reporting in Clinical Trials
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Clinical Trial Safety Reporting Requirements in the EU and USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Good Clinical Laboratory Practice
£69.00Original price was: £69.00.£49.00Current price is: £49.00. exc. VAT
Drug Safety and Pharmacovigilance
Our courses on drug safety and pharmacovigilance provide distinctive, high-quality presentation of crucial aspects of this vitally important field, which is the focus of ever more stringent regulation. The modules offer essential training for medical, safety, pharmacovigilance, and clinical research staff of pharma/biotech companies and contract research organisations; healthcare professionals and regulatory authority staff will also find them valuable.
-
An Introduction to Drug Safety and Pharmacovigilance
£149.00Original price was: £149.00.£139.00Current price is: £139.00. exc. VAT -
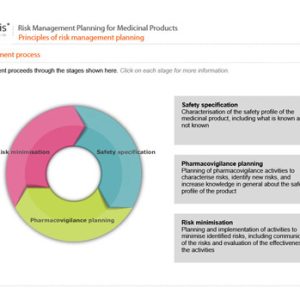
Risk Management Planning for Medicinal Products
£61.00Original price was: £61.00.£39.00Current price is: £39.00. exc. VAT -
Urgent Safety Restrictions
£37.00Original price was: £37.00.£25.00Current price is: £25.00. exc. VAT
Good Manufacturing Practice
-
Good Quality Control Laboratory Practice
£79.00Original price was: £79.00.£69.00Current price is: £69.00. exc. VAT -
Good Manufacturing Practice for the Warehouse
£74.00Original price was: £74.00.£49.00Current price is: £49.00. exc. VAT -
Good Practices (GxP) in Drug Development and Manufacturing
£29.00Original price was: £29.00.£25.00Current price is: £25.00. exc. VAT
Good Practices for Regulated Laboratories
Laboratories subject to inspection by regulatory authorities need to comply with international standards, regulatory guidance, and legal requirements as applicable. Such laboratories include those that carry out nonclinical studies and those performing quality control testing of products and materials in the manufacture of medicines. The former need to comply with Good Laboratory Practice (GLP); the latter need to meet the requirements of those aspects of Good Manufacturing Practice that we call Good Quality Control Laboratory Practice (GQCLP).
Our courses on GLP and GQCLP provide essential learning for all personnel of analytical laboratories, especially those working in test facilities undertaking nonclinical studies or in quality control of medicinal products.
-
Good Quality Control Laboratory Practice
£79.00Original price was: £79.00.£69.00Current price is: £69.00. exc. VAT -
Good Clinical Laboratory Practice
£69.00Original price was: £69.00.£49.00Current price is: £49.00. exc. VAT
Medical Devices
Our introductory course on medical devices provides an introduction to the basics of their regulation, especially the requirements that manufacturers must meet in order to market devices in Europe and the USA.
Our module explains what medical devices are and gives examples of the various types. It outlines the principles of their regulation and the criteria for placing them on the market, and it identifies major players in regulation worldwide.
It then outlines prominent characteristics of the regulation of medical devices in the USA and in Europe. The module is up to date with the current upheaval in European Union legislation on medical devices.
-
An Introduction to the Regulation of Medical Devices
£49.00Original price was: £49.00.£35.00Current price is: £35.00. exc. VAT
Information and Communication Technology
-
Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
£74.00Original price was: £74.00.£49.00Current price is: £49.00. exc. VAT -
Assuring Data Integrity in the Manufacture of Medicinal Products
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Assuring Data Integrity in Clinical Research
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT
Pharmacokinetics and Pharmacodynamics
Our courses on pharmacokinetic and pharmacodynamic studies provide a thorough grounding in this field of fundamental importance in the development and registration of medicinal products.
Pharmacokinetic (PK) and pharmacodynamic (PD) studies provide a bridge between science and medicine in the development of a drug.
Our first module describes the role of in-vivo PK and PD studies in a drug development programme, sets out the uses to which the findings can be put, and discusses their implications for clinical development and application for marketing approval.
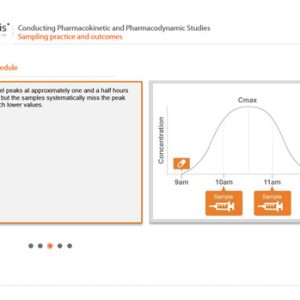
The other module extends the learner’s understanding of PK and PD studies from the basics described in PKPD01. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.
-
An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration
£74.00Original price was: £74.00.£49.00Current price is: £49.00. exc. VAT -
Conducting Pharmacokinetic and Pharmacodynamic Studies
£74.00Original price was: £74.00.£49.00Current price is: £49.00. exc. VAT
Regulatory Affairs & Compliance
-
How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
How to Conduct Clinical Research Under the EU Clinical Trials Regulation
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Safety Reporting in Clinical Trials
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Clinical Trial Safety Reporting Requirements in the EU and USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Essentials of EU and US Regulatory Affairs for Human Medicinal Products
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
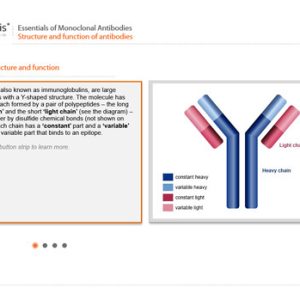
Essentials of Monoclonal Antibodies
£39.00Original price was: £39.00.£29.00Current price is: £29.00. exc. VAT -
Orphan Drug Designation in the USA and Europe
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
The European Centralised Procedure (CP)
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
The Mutual Recognition Procedure (MRP)
£98.00Original price was: £98.00.£75.00Current price is: £75.00. exc. VAT -
Electronic Common Technical Document (eCTD)
£126.00Original price was: £126.00.£89.00Current price is: £89.00. exc. VAT -
Variations to Marketing Authorisations in Europe
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
The New Drug Application (NDA) for Marketing Approval in the USA
£129.00Original price was: £129.00.£89.00Current price is: £89.00. exc. VAT -
The Decentralised Procedure (DCP)
£98.00Original price was: £98.00.£74.00Current price is: £74.00. exc. VAT -
Registration of Medicinal Products Based on Monoclonal Antibodies
£74.00Original price was: £74.00.£59.00Current price is: £59.00. exc. VAT -
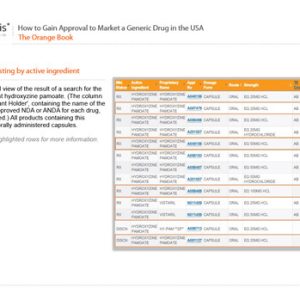
How to Gain Approval to Market a Generic Drug in the USA
£99.00Original price was: £99.00.£89.00Current price is: £89.00. exc. VAT -
The Biologics License Application (BLA) for Marketing Approval in the USA
£99.00Original price was: £99.00.£89.00Current price is: £89.00. exc. VAT -
The 505(b)(2) Application for Marketing Approval in the USA
£59.00Original price was: £59.00.£39.00Current price is: £39.00. exc. VAT
Sales and Marketing
-
Legal and Regulatory Framework for Advertising and Promotion of Prescription Drugs in the USA
£90.00Original price was: £90.00.£70.00Current price is: £70.00. exc. VAT -
Regulatory Requirements and Guidance on Advertising and Promotion of Prescription Drugs in the USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Consumer-directed Advertising and Online Promotion of Prescription Drugs in the USA
£90.00Original price was: £90.00.£60.00Current price is: £60.00. exc. VAT -
Marketing of Prescription Drugs in the USA – Interactions with Healthcare Professionals
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT
Validation
Our courses on validation provide essential training for manufacturing personnel in the medicines and healthcare products industries.
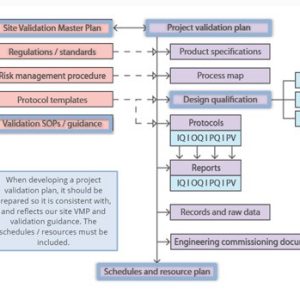
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. Our suite of courses provides an introduction to validation, and practical advice on equipment commissioning and qualification, cleaning validation, and computer systems validation.
-
Introduction to Validation
£99.00Original price was: £99.00.£69.00Current price is: £69.00. exc. VAT -
Validation Plans and Documentation
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
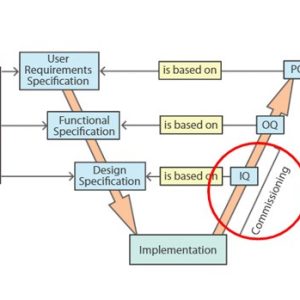
Commissioning and Installation Qualification
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
Operational and Performance Qualification
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
Equipment Cleaning Validation
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
Computer Systems Validation, Part 1: Planning
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
Computer Systems Validation, Part 2: Implementation
£99.00Original price was: £99.00.£69.00Current price is: £69.00. exc. VAT
Micro Courses
-
Clinical trial monitor’s GCP responsibilities
£25.00Original price was: £25.00.£20.00Current price is: £20.00. exc. VAT -
Data capture and management in clinical trials
£25.00Original price was: £25.00.£15.00Current price is: £15.00. exc. VAT