Data capture and management in clinical trials
Course Summary
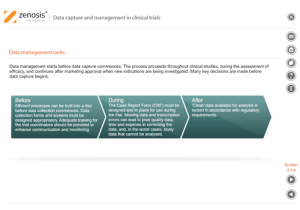
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
£25.00 Original price was: £25.00.£15.00Current price is: £15.00. exc. VAT
Purchasing Information
When you have completed your course order, Zenosis will finalise the setup of your course materials and contact you on the email address that you provide in your order. You can expect this process to be completed within one business day (using the UK business calendar) of completing your payment.
It is therefore essential that you use your real email address for your order, or indicate in the purchase notes the email address to be used for the course set-up, and check that any messages from Zenosis or grapl are not lost in your junk or spam folder.
You will have access to the course module(s) for a period of 180 days after your purchase is complete.
Detailed Course Information
• Describe the purpose of data capture and management
• Outline the regulatory requirements for clinical data capture and management
• Describe how electronic data capture can improve data quality and shorten trial timelines
This module is intended for all those involved in the preparation, design, conduct or analysis of clinical trials. It will be useful to new entrants to the field or as a refresher for staff, including clinical research associates and data managers, in the clinical/medical departments of pharmaceutical or biotechnology companies or in contract research organisations. It will also be of interest to clinical investigators, study coordinators, and other healthcare staff working on clinical trials.
