Principles of GMP
Course Summary
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
Purchasing Information
When you have completed your course order, Zenosis will finalise the setup of your course materials and contact you on the email address that you provide in your order. You can expect this process to be completed within one business day (using the UK business calendar) of completing your payment.
It is therefore essential that you use your real email address for your order, or indicate in the purchase notes the email address to be used for the course set-up, and check that any messages from Zenosis or grapl are not lost in your junk or spam folder.
You will have access to the course module(s) for a period of 180 days after your purchase is complete.
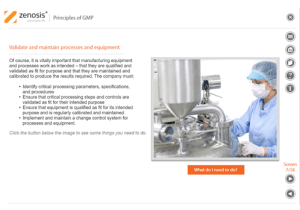
Detailed Course Information
• Describe ways of preventing contamination
• Outline precautions to prevent mix-ups
• Describe requirements for documentation
• List steps taken to validate and maintain processes and equipment
• Identify requirements for an independent Quality unit and self-inspection
• Outline training requirements
• Discuss the roles of quality assurance, GMP, and quality control in the company’s quality management system
Everyone who works in, or has occasion to enter into, a manufacturing environment in the pharma/biotech industry should have access to this module.
