PRICING AND DISCOUNTS
VAT is added for UK purchases at checkout
| Purchase | Discount |
|---|---|
| 1 course | 0% |
| 2 courses | 5% |
| 3 courses | 10% |
| 4 courses | 15% |
| 5 courses | 20% |
| more(each) | 25% |
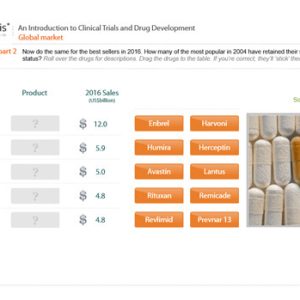
Clinical Trials
Our courses on clinical trials cover the design, set-up, monitoring and audit/inspection of trials.
We offer modules providing extensive training for clinical research associates and other staff of pharma/biotech or contract research organisations involved in clinical development. We also offer modules of value both to such staff and to clinical investigators.
At a time of upheaval in the legislation on clinical research, our continually updated courses provide an essential guide to requirements and best practice.
“ICH E6(R3) Good Clinical Practice” has been added to your basket. View basket
-
ICH E6(R3) Good Clinical Practice
£159.00Original price was: £159.00.£99.00Current price is: £99.00. exc. VAT -
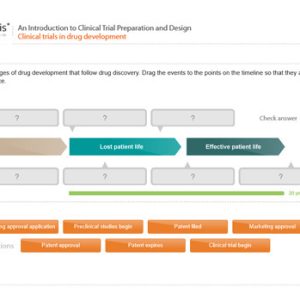
An Introduction to Clinical Trial Preparation and Design
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
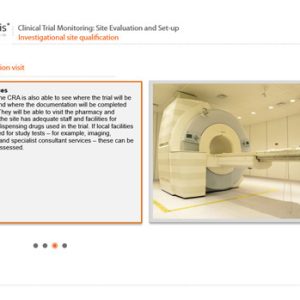
Clinical Trial Monitoring: Site Evaluation and Set-up
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
£98.00Original price was: £98.00.£75.00Current price is: £75.00. exc. VAT -
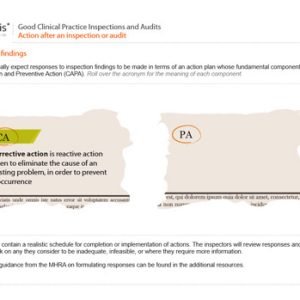
Good Clinical Practice Inspections and Audits
£126.00Original price was: £126.00.£89.00Current price is: £89.00. exc. VAT -
The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
How to Conduct Clinical Research Under the EU Clinical Trials Regulation
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Safety Reporting in Clinical Trials
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Clinical Trial Safety Reporting Requirements in the EU and USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Good Clinical Laboratory Practice
£69.00Original price was: £69.00.£49.00Current price is: £49.00. exc. VAT