PRICING AND DISCOUNTS
VAT is added for UK purchases at checkout
| Purchase | Discount |
|---|---|
| 1 course | 0% |
| 2 courses | 5% |
| 3 courses | 10% |
| 4 courses | 15% |
| 5 courses | 20% |
| more(each) | 25% |
Clinical Trials
Our courses on clinical trials cover the design, set-up, monitoring and audit/inspection of trials.
We offer modules providing extensive training for clinical research associates and other staff of pharma/biotech or contract research organisations involved in clinical development. We also offer modules of value both to such staff and to clinical investigators.
At a time of upheaval in the legislation on clinical research, our continually updated courses provide an essential guide to requirements and best practice.
-
ICH E6(R3) Good Clinical Practice
£159.00Original price was: £159.00.£99.00Current price is: £99.00. exc. VAT -
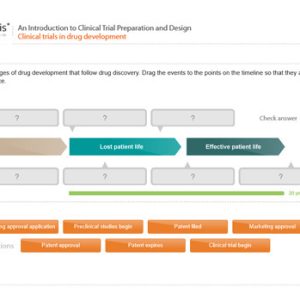
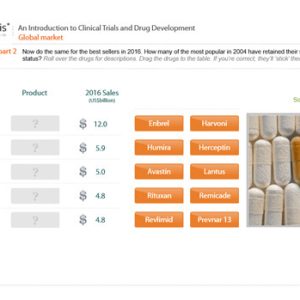
An Introduction to Clinical Trial Preparation and Design
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
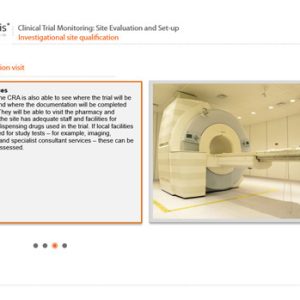
Clinical Trial Monitoring: Site Evaluation and Set-up
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
£98.00Original price was: £98.00.£75.00Current price is: £75.00. exc. VAT -
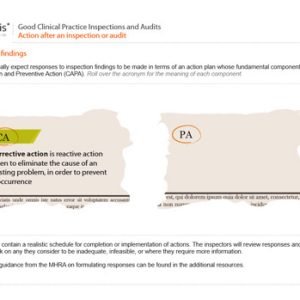
Good Clinical Practice Inspections and Audits
£126.00Original price was: £126.00.£89.00Current price is: £89.00. exc. VAT -
The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
How to Conduct Clinical Research Under the EU Clinical Trials Regulation
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Safety Reporting in Clinical Trials
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Clinical Trial Safety Reporting Requirements in the EU and USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Good Clinical Laboratory Practice
£69.00Original price was: £69.00.£49.00Current price is: £49.00. exc. VAT