PRICING AND DISCOUNTS
VAT is added for UK purchases at checkout
| Purchase | Discount |
|---|---|
| 1 course | 0% |
| 2 courses | 5% |
| 3 courses | 10% |
| 4 courses | 15% |
| 5 courses | 20% |
| more(each) | 25% |
Regulatory Affairs & Compliance
Our courses on regulatory affairs and compliance cover the main submissions required for the development and marketing of medicinal products in Europe and the USA. These include applications for approval to conduct clinical trials, to be granted orphan product status, to market new or generic products, and to make post-marketing changes. Whether they are introductory courses for entry-level regulatory affairs and for support staff, or they are more advanced courses for more experienced staff, they provide both a training and a reference tool that is continually updated in response to developments in regulatory requirements.
-
The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
£149.00Original price was: £149.00.£99.00Current price is: £99.00. exc. VAT -
How to Gain Authorisation for Clinical Research Under the EU Clinical Trials Regulation
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
How to Conduct Clinical Research Under the EU Clinical Trials Regulation
£49.00Original price was: £49.00.£39.00Current price is: £39.00. exc. VAT -
Safety Reporting in Clinical Trials
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Clinical Trial Safety Reporting Requirements in the EU and USA
£95.00Original price was: £95.00.£75.00Current price is: £75.00. exc. VAT -
Essentials of EU and US Regulatory Affairs for Human Medicinal Products
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
Essentials of Monoclonal Antibodies
£39.00Original price was: £39.00.£29.00Current price is: £29.00. exc. VAT -
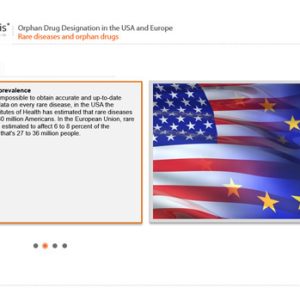
Orphan Drug Designation in the USA and Europe
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
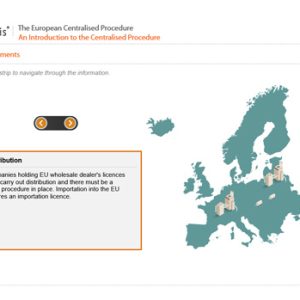
The European Centralised Procedure (CP)
£74.00Original price was: £74.00.£69.00Current price is: £69.00. exc. VAT -
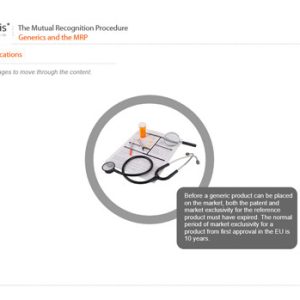
The Mutual Recognition Procedure (MRP)
£98.00Original price was: £98.00.£75.00Current price is: £75.00. exc. VAT -
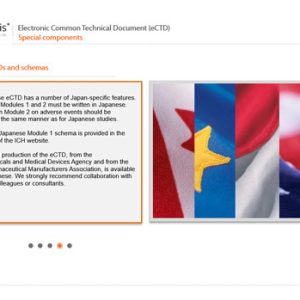
Electronic Common Technical Document (eCTD)
£126.00Original price was: £126.00.£89.00Current price is: £89.00. exc. VAT -
Variations to Marketing Authorisations in Europe
£99.00Original price was: £99.00.£79.00Current price is: £79.00. exc. VAT -
The New Drug Application (NDA) for Marketing Approval in the USA
£129.00Original price was: £129.00.£89.00Current price is: £89.00. exc. VAT -
The Decentralised Procedure (DCP)
£98.00Original price was: £98.00.£74.00Current price is: £74.00. exc. VAT -
Registration of Medicinal Products Based on Monoclonal Antibodies
£74.00Original price was: £74.00.£59.00Current price is: £59.00. exc. VAT -
How to Gain Approval to Market a Generic Drug in the USA
£99.00Original price was: £99.00.£89.00Current price is: £89.00. exc. VAT -
The Biologics License Application (BLA) for Marketing Approval in the USA
£99.00Original price was: £99.00.£89.00Current price is: £89.00. exc. VAT -
The 505(b)(2) Application for Marketing Approval in the USA
£59.00Original price was: £59.00.£39.00Current price is: £39.00. exc. VAT